Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is approved to treat certain psychiatric conditions, including major depressive disorder and obsessive compulsive disorder.

About Tms
-
Non-drug treatment option
-
Non-invasive
-
FDA Approved
-
Covered by most insurance plans
-
Available for patients ages 18-70 years old
-
Select insurance coverage for age 15+

Treatment efficacy
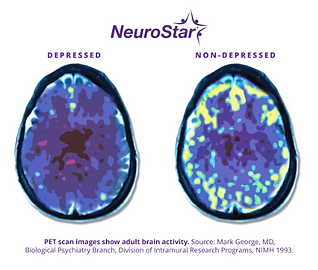
TMS has been available since 2018 and has been shown to significantly reduce the symptoms of certain psychiatric illnesses. TMS can be used on its own or along with medication management. During a NeuroStar TMS treatment session, a magnet similar in strength to that used in a magnetic resonance imaging (MRI) machine is used to stimulate nerve cells in the area of the brain thought to control negative psychiatric symptoms.
This non-medication treatment option essentially jumpstarts areas in our brain with reduced neuronal activity, which causes our negative psychiatric symptoms, with efficacy ongoing even after TMS treatment sessions are complete, making long term remission possible.
A study of real-world outcomes reported
an 83% measurable improvement in symptoms & a 62% remission rate.
